Abstract
Background: Advances in the treatment of hemophilia have resulted in a nearly normal life expectancy for persons with hemophilia (PwH). As a result of this improved life expectancy the aging hemophilia population is now experiencing age-related complications including cardiovascular disease (CVD), which is the leading cause of death in the US general population. Several studies have shown that the CVD risk profile among PwH is less favorable than that in the general population (Fransen van de Putte et al., 2013; Sood et al., 2018). Despite higher rates of hypertension among PwH, rates of treatment are lower than in the general population (Sood et al., 2018). We also know that atherosclerosis develops at the same rate among PwH as in the general population (Biere-Rafi et al., 2012; Zwiers et al., 2012). When CVD occurs in PwH, the management is more complicated due to the requirement for clotting factor concentrates to offset anticoagulant and antiplatelet therapies and prevent serious bleeding complications (Martin & Key, 2016). Recent prospective data indicate that the incidence of cardiovascular endpoints in PwH is lower than would be predicted by the QRISK-2011 score, but the use of novel therapies was not analyzed in that study, and we do not know how widespread use of these therapies will impact the development of CVD among PwH (Van Der Valk et al., 2022). Primary prevention of CVD through risk factor management is essential to prevent cardiovascular complications in the era of novel therapies for hemophilia.
Study Design and Methods:
This multicenter observational cohort study will utilize quality improvement science informed by frequent data sampling to improve the management of hypertension and dyslipidemia in PwH. Ten large hemophilia treatment centers will contribute 100 PwH each and will be stratified by age such that 25% of the population will be age 18-29, 30-44, 45-59, and 60-89. PwH will be eligible for inclusion if they have hemophilia A or B of any severity, are age 18-89, have been seen in the last 2 years, and do not anticipate relocating in the next 3 years. Men and women with hemophilia will be included. Individual consent will not be required as the dataset will be completely deidentified and the quality improvement interventions will be applied broadly to the hemophilia population at each center. Data will be collected from routine clinical care records. The primary outcome will be the prevalence of diagnosed and undiagnosed hypertension in this cohort. Secondary outcomes will be prevalence of dyslipidemia and rates of treatment for hypertension and dyslipidemia. Exploratory and descriptive statistical analyses will be performed.
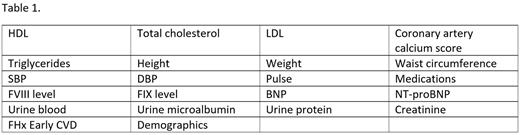
Deidentified data will be entered into a database via electronic medical record integration. This will allow for collection of 5 years of retrospective data, and subsequent monthly prospective data collection for 3 years. The automated nature of the data collection is novel and will allow for rapid record collection with minimal manual data entry, which can introduce errors and delay analysis. As such, a substantial number of records and timepoints can be collected. Conventional manual data entry of this scale would not be feasible for many independent academic studies. Table 1 contains the most relevant variables that will be collected.
Retrospective data will be analyzed to determine the prevalence of hypertension, dyslipidemia, and established CVD. Rates of hypertension and dyslipidemia meeting criteria for diagnosis, but without an official diagnosis will also be determined along with rates of treatment in those who have been diagnosed. Investigators will be trained on the latest guidelines from the American College of Cardiology for the management of hypertension and dyslipidemia as part of this initiative. A survey will be distributed to participating investigators to determine barriers to control of vascular risk factors. This data be used by a steering committee to guide quality improvement efforts at each site. Monthly reports will inform investigators on the progress of their quality improvement initiatives.
Disclosures
Hardesty:Novartis: Other: Publication Assistance; Global Blood Therapeutics: Research Funding; Biomarin: Membership on an entity's Board of Directors or advisory committees, Other: Publication Assistance; Novo Nordisk: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Publication Assistance; Takeda: Membership on an entity's Board of Directors or advisory committees, Other: Publication Assistance; Bayer: Research Funding. Sood:Bayer: Membership on an entity's Board of Directors or advisory committees. Escobar:Takeda: Honoraria; Novo Nordisk: Honoraria; Sanofi: Honoraria; UniQure: Honoraria; Genentech: Honoraria; CSL Behring: Honoraria; Bayer: Honoraria; Kedrion: Honoraria; Hemobiologics/LFB: Honoraria; The National Hemophilia Foundation: Honoraria; Pfizer: Honoraria; BioMarin: Honoraria. Quon:Novo Nordisk: Consultancy, Honoraria, Other: travel support , Speakers Bureau; Octapharma: Consultancy; Bayer: Consultancy; BioMarin Pharmaceutical Inc.: Consultancy, Honoraria, Other: clinical trial investigator, travel support , Speakers Bureau; Roche/Genentech: Consultancy, Honoraria, Other: Clinical trial investigator, travel support , Speakers Bureau; Takeda: Consultancy, Honoraria, Other: Clinical trial investigator, travel support, Speakers Bureau; Sanofi: Consultancy, Honoraria, Other: Clinical trial investigator, travel support , Speakers Bureau; UniQure: Other: Clinical trial investigator.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal